Copper ii sulfate mass
Home » chemical » Copper ii sulfate mass >Copper ii sulfate mass
Copper Ii Sulfate Mass. Long-term exposure in rats and mice showed no overt signs of toxicity other than a dose-related reduction in growth. Ii A brown mass gets deposited around the nail. Death is preceded by gastric hemorrhage tachycardia hypotension hemolytic crisis convulsions and paralysis. Crystals of copperII chloride in a container It is made by reacting copper with chlorine.
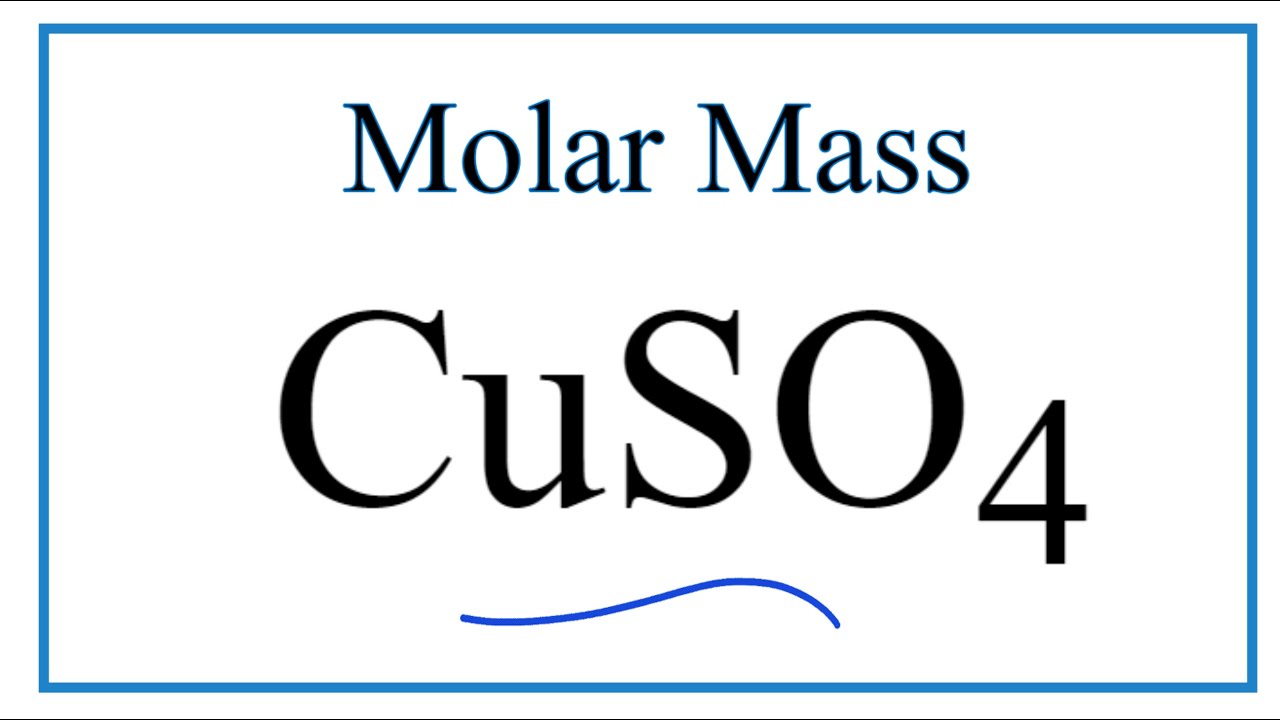 Molar Mass Molecular Weight Of Cuso4 Copper Ii Sulfate Youtube From youtube.com
Molar Mass Molecular Weight Of Cuso4 Copper Ii Sulfate Youtube From youtube.com
So here on adding zinc to CuSO4 solution zinc displaces copper from copper sulphate forms zinc sulphate solution. 63546 32065 1599944 Percent composition by element. The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water. This dark blue to purple solid is a salt of the metal complex CuNH 3 4 H 2 O 2It is closely related to Schweizers reagent which is used for the production of cellulose fibers in the production of rayon. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form. What is the molecular equation of copper II sulfate barium chloride.
The waters of hydration are released from the solid crystal and form water vaporThe hydrated form is medium blue and the dehydrated solid is light blue.
CopperII sulfate solution CuSO 4 aq see CLEAPSS Hazcard HC027c and CLEAPSS Recipe Book RB031. This is a class experiment suitable for students who already have a reasonable understanding of the mole concept. Divide each mass by the atomic mass of the element to give moles K 182 391 I 5931269 O 22416 00465 mol 00467mol 014mol. The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water. This information is used to find x in the formula CuSO 4xH 2 O using mole calculations. CopperII Hydroxide Cu2O CopperI Oxide Cu2S CopperI Sulfide Cu3PO42 CopperII Phosphate CuBr CopperI Bromide CuCl CopperI Chloride CuCl2 CopperII Chloride CuCO3 CopperII Carbonate CuFeS2 Chalcopyrite CuI CopperI Iodide CuNO3 CopperI Nitrate CuO CopperII Oxide CuS CopperII Sulfide CuSO4 CopperII Sulfate CuSO45H2O.
 Source: researchgate.net
Source: researchgate.net
5 Related Records Expand this. The balanced equation is. CuSO4 BaCl2 –. It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction. For example copperII pentahydrate CuSO_4 5H_2O is blue.
 Source: youtube.com
Source: youtube.com
This is indicated by colour change from blue to colourless. Long-term exposure in rats and mice showed no overt signs of toxicity other than a dose-related reduction in growth. When copperII sulfate pentahydrate CuSO45H2O is heated it decomposes to the dehydrated form. 1 Structures Expand this section. If you are a moderator please see our troubleshooting guide.
 Source: youtube.com
Source: youtube.com
CopperII sulfate solution is HARMFUL if concentration is equal to or greater than 1 M. Can be calculated from the ratio of moles. What happens when iron fillings are added to sulphate solution. CopperII sulfate solution is HARMFUL if concentration is equal to or greater than 1 M. On the other hand.
 Source: youtube.com
Source: youtube.com
When copperII sulfate pentahydrate CuSO45H2O is heated it decomposes to the dehydrated form. CuCl2 BaSO4 What is the chemical equation for the reaction between silver. So here on adding zinc to CuSO4 solution zinc displaces copper from copper sulphate forms zinc sulphate solution. Ii A brown mass gets deposited around the nail. This information is used to find x in the formula CuSO 4xH 2 O using mole calculations.

It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction. Death is preceded by gastric hemorrhage tachycardia hypotension hemolytic crisis convulsions and paralysis. It has a role as a sensitiser a fertilizer and an emetic. If the concentrations are increased the solutions must be labelled with the correct hazard warnings. Divide each mass by the atomic mass of the element to give moles K 182 391 I 5931269 O 22416 00465 mol 00467mol 014mol.
 Source: youtube.com
Source: youtube.com
Since the only component other than H2O and Cu 2 is the sulfate ion SO 4 2 we can now determine the complete formula of the hydrated copperII sulfate. O and magnesium sulfate MgSO. CopperII Mg2 magnesium Ni2 nickelII Sr2 strontium Zn2 zinc Sn2 tinII Pb 2 leadII Ba2 barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3-hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3 phosphate Use the ions given above to. Death is preceded by gastric hemorrhage tachycardia hypotension hemolytic crisis convulsions and paralysis. Mass is the quantity of matter present.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1 Structures Expand this section. It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction. Anhydrous CuSO 4 has a grey-white powdery appearance whereas the pentahydrate has a bright blue colour. Divide each mass by the atomic mass of the element to give moles K 182 391 I 5931269 O 22416 00465 mol 00467mol 014mol. CopperII Mg2 magnesium Ni2 nickelII Sr2 strontium Zn2 zinc Sn2 tinII Pb 2 leadII Ba2 barium Fe3 ironIII Al3 aluminium Cr3 ChromiumIII F-fluoride Cl-chloride Br-bromide I-iodide HO-hydroxide NO 2-nitrite NO 3-nitrate HCO 3-hydrogencarbonate O2-oxide CO 3 2-carbonate SO 4 2-sulfate HSO 4-hydrogensulfate S2-sulphide N3-nitride PO 4 3 phosphate Use the ions given above to.

O and magnesium sulfate MgSO. CuSO4 BaCl2 –. When copperII sulfate pentahydrate CuSO45H2O is heated it decomposes to the dehydrated form. Now the difference between hydrated copperII sulfate and anhydrous copperII sulfate except for the fact that the former contains water of crystallization is the color. This is a class experiment suitable for students who already have a reasonable understanding of the mole concept.
 Source: youtube.com
Source: youtube.com
It can also be made by reacting copperII hydroxide copperII oxide or copperII carbonate with hydrochloric acid and from pure copper and from 11 solution of hydrogen peroxide and hydrochloric acid where copper first get oxidized to CuO from H2O2 and then reacts with HCl to form CuCl2 reaction. CopperII sulfate solution CuSO 4 aq see CLEAPSS Hazcard HC027c and CLEAPSS Recipe Book RB031. We were unable to load Disqus. Atomic Mass of Atoms. In the case of copperII sulfate hydrates you only get x as a whole number.
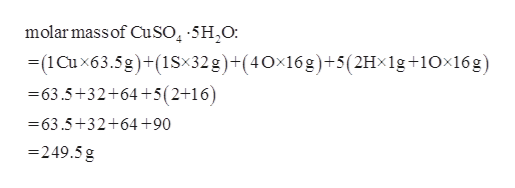 Source: bartleby.com
Source: bartleby.com
The pentahydrate CuSO 4 5H 2 O the most commonly encountered salt is bright blue. What is the molecular equation of copper II sulfate barium chloride. At the suggested concentrations the copperII sulfate solution is LOW HAZARD. CopperII Sulfate molecular weight. If you are a moderator please see our troubleshooting guide.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title copper ii sulfate mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.