Copper i sulfate formula
Home » chemical » Copper i sulfate formula >Copper i sulfate formula
Copper I Sulfate Formula. Convert grams CopperII Sulfate to moles or moles CopperII Sulfate to grams. Molecular FormulaCuO4S5H2O Molecular Weight24968 Section 10 - Stability and Reactivity Chemical Stability. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. When it is mixed with calcium hydroxide it is known as Bordeaux mixture.
 How To Write The Formula For Copper I Sulfate Youtube From youtube.com
How To Write The Formula For Copper I Sulfate Youtube From youtube.com
It is produced industrially by treating copper metal with hot concentrated sulfuric. It can also be made by electrolyzing a solution of sodium bicarbonate with a copper anode. It is sprayed on plants as a preventive treatment. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species. The body stores copper mostly in the bones and muscles. Vapor pressure mmHg at 25 C 1.
It releases chlorine and turns into copperI chloride when heated very hot.
Its mode of action is ineffective after a fungus has become established. For instance the copper sulfate used earlier in the semester was stated to be CuSO 4 but it actually had absorbed 5 moles of water for every 1 mole of CuSO 4 and should have been correctly labeled as CuSO 45H 2O copper II sulfate pentahydrate. The simplest ion that copper forms in solution is the typical blue hexaaquacopperII. As CuSO 4 is an electrolyte it splits into Cu cation and SO 4 anion ions and move freely in the solution. The names are found by finding the intersection between the cations and anions. Copper sulfate pentahydrate CuSO45H2O or CuH10O9S CID 24463 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
 Source: youtube.com
Source: youtube.com
The most common form of copper sulfate is its pentahydrate given by the chemical formula CuSO 45H 2 O. A strong base is a base that is completely dissociated in an aqueous solutionThese compounds ionize in water to yield one or more hydroxide ion OH- per molecule of base. B the chemical formula of the compound appears after the arrow. Older names for this compound include blue vitriol bluestone vitriol of copper and Roman vitriol. Reactions of copperII ions in solution.

It reacts with sodium hydroxide to make copperII hydroxide. Because of the presence of copper the compound has a characteristic blue. Royal Society of Chemistry. It releases chlorine and turns into copperI chloride when heated very hot. E-mail to a friend.

However it can be noted that the anhydrous form of this salt is a powder that is white. Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. The simplest ion that copper forms in solution is the typical blue hexaaquacopperII. Older names for this compound include blue vitriol bluestone vitriol of copper and Roman vitriol. Sodium sulfate Na 2 SO 4.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Its mode of action is ineffective after a fungus has become established. Stable at room temperature in closed containers under normal storage and handling conditions. Product Name CopperII sulfate Cat No. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner. Copper sulfate pentahydrate CuSO45H2O or CuH10O9S CID 24463 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
 Source: softschools.com
Source: softschools.com
Copper sulfate pentahydrate CuSO45H2O or CuH10O9S CID 24463 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Molecular weight gmol 3. This page looks at some aspects of copper chemistry required for UK A level exams. The loss in mass of the compound after. Copper is a mineral.
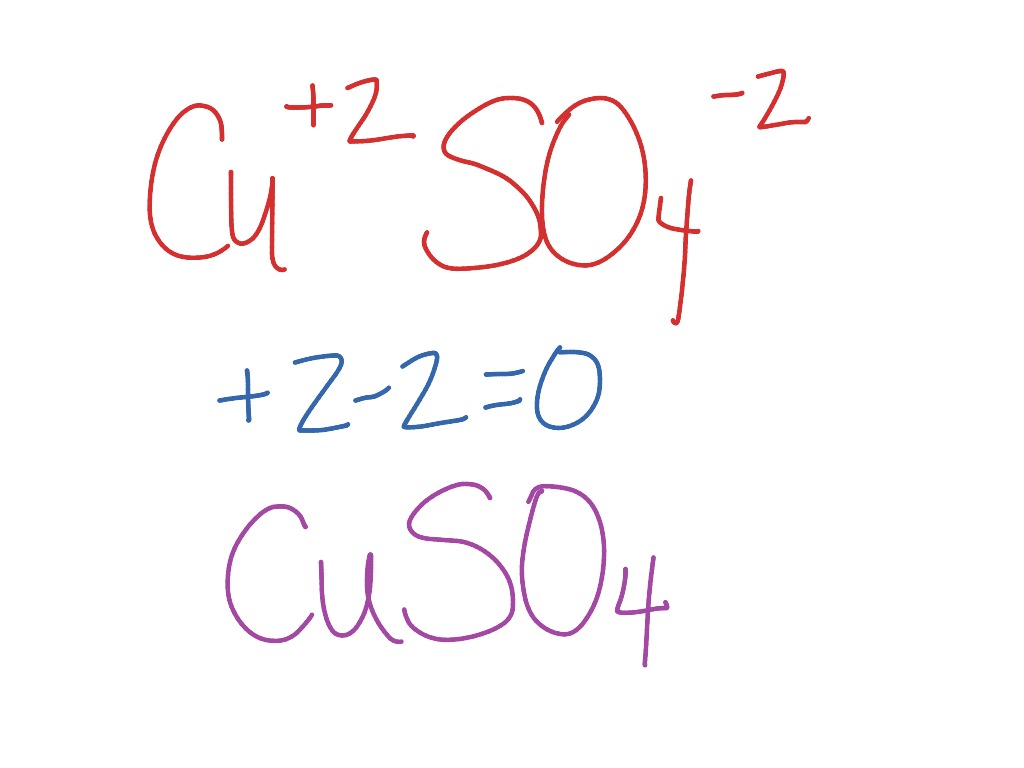 Source: showme.com
Source: showme.com
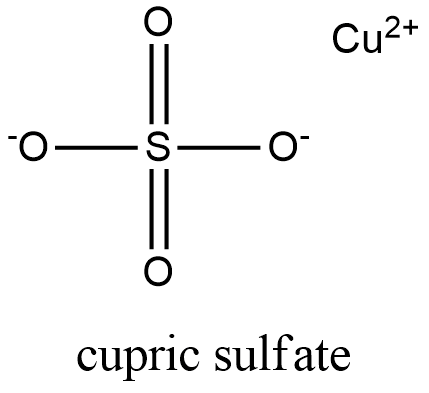
Sodium sulfate 2Na SO 4 2- Na 2 SO 4. Whenever copper sulfate or CuSO 4 is added to water it gets dissolved in the water. Sodium sulfate Na 2 SO 4. However it can be noted that the anhydrous form of this salt is a powder that is white. CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In this video well write the correct formula for Copper II sulfate CuSO4To write the formula for Copper II sulfate well use the Periodic Table a Com. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment. CopperII hydroxide can be made by reacting copper sulfate with sodium hydroxide. CopperII sulfateVI-5-water CuSO 45H 2 Os HARMFUL DANGEROUS FOR THE ENVIRONMENT see CLEAPSS Hazcard HC027c. A strong base is a base that is completely dissociated in an aqueous solutionThese compounds ionize in water to yield one or more hydroxide ion OH- per molecule of base.
 Source: youtube.com
Source: youtube.com
CuSO 4 5H 2 O. E-mail to a friend. Reactions of copperII ions in solution. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner. The loss in mass of the compound after.
 Source: youtube.com
Source: youtube.com
Copper sulfate also known as blue vitriol Salzburg vitriol Roman vitriol blue copperas or bluestone is a chemical compound comprised of Copper Sulphur and Oxygen whose formula is CuSO4. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. The Cu ions cation will be attracted towards cathode ie. Royal Society of Chemistry. Uses advised against Food drug pesticide or biocidal product use.
 Source: youtube.com
Source: youtube.com
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. A strong base is a base that is completely dissociated in an aqueous solutionThese compounds ionize in water to yield one or more hydroxide ion OH- per molecule of base. The most common form of copper sulfate is its pentahydrate given by the chemical formula CuSO 45H 2 O. Royal Society of Chemistry. Because of the presence of copper the compound has a characteristic blue.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title copper i sulfate formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.