Cobalt fluoride solubility
Home » chemical » Cobalt fluoride solubility >Cobalt fluoride solubility
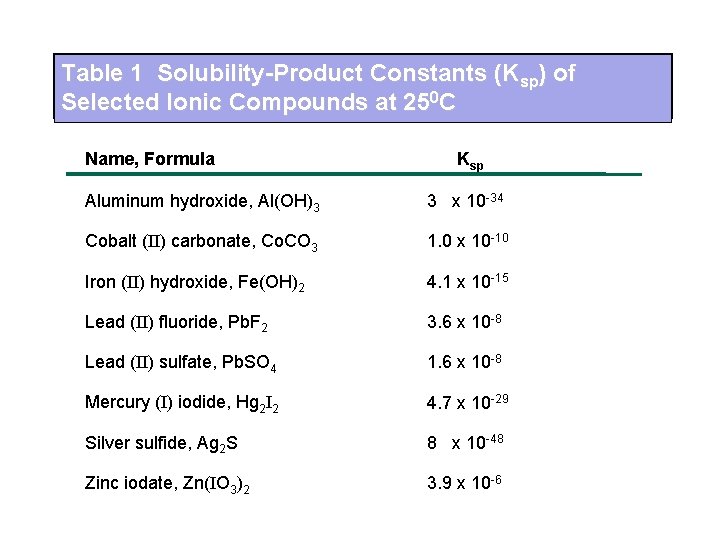
Cobalt Fluoride Solubility. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. 56 gL 0 C 67 gL 20 C 172 gL 100 C.
 Cobalt Iii Fluoride Wikipedia From en.wikipedia.org
Cobalt Iii Fluoride Wikipedia From en.wikipedia.org
Although odorless lithium fluoride has a bitter-saline taste. Vapour pressure is a measure of the ability of a compound to bond with itself. Rubidium fluoride copperIIsulfate Solubility Rules. The Journal of Prosthetic Dentistry is the leading professional journal devoted exclusively to prosthetic and restorative dentistryThe Journal is the official publication for 24 leading US. Less solubilty of NaHCO 3 is very important factor in sodium carbonate manufacturing process by solvay process. Cobalt chloride as an example of a solid hydrate.
CdC 2 O 4 x 3H 2 O.
Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n. 56 gL 0 C 67 gL 20 C 172 gL 100 C. Table PageIndex2 lists the names of some common monatomic ions. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Cobalt chloride can be used to test for chloride ions in this way. Epaminondas Voutsas in Thermodynamics Solubility and Environmental Issues 2007.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Less solubilty of NaHCO 3 is very important factor in sodium carbonate manufacturing process by solvay process. K sp M n m A m- n. The anhydrous without water blue form can be made by reacting cobalt with chlorine. The name of a monatomic anion consists of the stem of the element name the suffix -ide and then the word ionThus as we have already seen Cl is chlor- -ide ion or the chloride ion. Pressure can also affect solubility but only for gases that are in liquids.
Source:
Table PageIndex2 lists the names of some common monatomic ions. A colorful example is cobaltII chloride which turns from blue to red upon hydration and can therefore be used as a water indicator. K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. Its structure is analogous to that of sodium chloride but it is much less soluble in water. Barium nitrate ammonium phosphate 11.
 Source: researchgate.net
Source: researchgate.net
Precipitates of alkaline earth metals. Vapour pressure of a pure compound is the pressure characteristic at any given temperature of a vapour in equilibrium with its liquid or solid form. Anhydrous cobalt chloride upper left and its crystal lattice structure lower left compared with cobalt chloride hexahydrate upper right and its. Thus if K sp Mm An is the equilibrium. A colorful example is cobaltII chloride which turns from blue to red upon hydration and can therefore be used as a water indicator.
 Source: guidechem.com
Source: guidechem.com
56 gL 0 C 67 gL 20 C 172 gL 100 C. K sp M n m A m- n. Rubidium fluoride copperIIsulfate Solubility Rules. Table PageIndex2 lists the names of some common monatomic ions. The maximum amount.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Alkaline earth metals forms both precipitates and solutions. Barium nitrate ammonium phosphate 11. Henrys law states that the solubility of a gas is directly proportional to the partial pressure of the gas. Alkaline earth metals forms both precipitates and solutions. Its structure is analogous to that of sodium chloride but it is much less soluble in water.
 Source: pubs.rsc.org
Source: pubs.rsc.org
Compound molecules that bond well with each other will. Vapour pressure is a measure of the ability of a compound to bond with itself. CdC 2 O 4 x 3H 2 O. Cobalt is used to produce alloys used in the manufacture of aircraft engines magnets grinding and cutting tools artificial hip and knee joints. Ag aq CH 3 COOHaq — AgCH 3 COOs H aq Nitrate is the only spectator ion.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Lets consider the saturated solution of silver chloride AgCl. Similarly O 2 is the oxide ion Se 2 is the selenide ion and so forth. Table PageIndex2 lists the names of some common monatomic ions. All salts of nitrates chlorates and acetates are soluble. A colorful example is cobaltII chloride which turns from blue to red upon hydration and can therefore be used as a water indicator.
Source: chemspider.com
Cobalt chloride can be used to test for chloride ions in this way. Vapour pressure is a measure of the ability of a compound to bond with itself. K_sp is called solubility product constant or simply solubility productIn general the solubility product of a compound represents the product of molar concentrations of ions raised to the power of their respective stoichiometric coefficients in the equilibrium reaction. Less solubilty of NaHCO 3 is very important factor in sodium carbonate manufacturing process by solvay process. It is a colorless solid that transitions to white with decreasing crystal size.
Anhydrous cobalt chloride upper left and its crystal lattice structure lower left compared with cobalt chloride hexahydrate upper right and its. It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. Compound molecules that bond well with each other will. Silver acetate is insoluble and you learn this from a solubility chart. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Source: slidetodoc.com
Source: slidetodoc.com
Thus if K sp Mm An is the equilibrium. Cobalt is used to produce alloys used in the manufacture of aircraft engines magnets grinding and cutting tools artificial hip and knee joints. Compound molecules that bond well with each other will. Cobalt chloride as an example of a solid hydrate. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title cobalt fluoride solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.