Cobalt 2 nitrate
Home » chemical » Cobalt 2 nitrate >Cobalt 2 nitrate
Cobalt 2 Nitrate. Pore size 02 μm pore size 05 μm cartridge nominal length 5 in. Page 1 of 1. Ammonia synthesis through electrochemical nitrate reduction was summarized. To understand the concerns over contamination in fish and how to reduce your exposure visit our Fish Information page.
 Cobalt Ii Nitrate Stock Image C043 8941 Science Photo Library From sciencephoto.com
Cobalt Ii Nitrate Stock Image C043 8941 Science Photo Library From sciencephoto.com
Hydroxyl Terminated Polybutadine HTPB Isodecyl Pelargonate IDP Isophorone di. Ammonia synthesis through electrochemical nitrate reduction was summarized. Co NO 3 H NO H 2O Co 2 a. 2 H 2O 10. Dimeryl-di-isocyanate DDI Dioctyl Adipate DOA Glycerin Glycerol. HX-878 Tepanol Hydroxyethyl Cellulose.
To understand the concerns over contamination in fish and how to reduce your exposure visit our Fish Information page.
2 H 2O d. 125 cm Code 7 EPDM rubber seal. 2 H 2O d. Compared with a single metal alloy metal. Package 2 Arsenic Uranium Lead NitrateNitrite Fluoride 5139 Note that the requisition form in the Halifax area and West. 1 chelating agents are organic compounds which wrap around a metal ion thanks to cationic side chains which form.
 Source: youtube.com
Source: youtube.com
1 chelating agents are organic compounds which wrap around a metal ion thanks to cationic side chains which form. CobaltII hydroxide precipitates as a solid when an alkali metal hydroxide is added to an aqueous solution of Co 2 salt. None of the above c. This is our newest publication and has been created to support the school technician profession in Scotland. Total Coliform Ecoli MPN Most Probable Number 4000.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
This is a chart of the most common charges for atoms of the chemical elements. 24 Dinitrotoluene DNT Barium Chromate. Page 1 of 1. Cobalt2 nitrate–water 16 AKOS025243254. Largemouth and smallmouth bass.

On the other hand cobaltIII chlorides can be obtained if the cobalt is bound also to other. For example Co 2 2 NaOH CoOH 2 2 Na The compound can be prepared by reacting cobaltII nitrate in water with a solution of triethylamine NC 2 H 5 3 as both the base and a complexing agent. Ionic bonds also melt at high temperatures. You can use this chart to predict whether or not an atom can bond with another atomThe charge on an atom is related to its valence electrons or oxidation stateAn atom of an element is most stable when its outer electron shell is completely filled or half-filled. None of the above c.

How many electrons are transferred and what would be the coefficient for H 2O in the balanced reaction. Cobalt is one of many metals that can be oxidized by nitric acid. 2 H 2O d. CobaltII hydroxide precipitates as a solid when an alkali metal hydroxide is added to an aqueous solution of Co 2 salt. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español.
 Source: sciencephoto.com
Source: sciencephoto.com
-nitrate NO 3-bromide Br - nitrite NO 2-chlorate ClO 3-perchlorate ClO 4-chlorite ClO 2 - permanganate MnO 4-chloride. CO 2 is trapped as Na 2 CO 3 on Ascarite. 1 chelating agents are organic compounds which wrap around a metal ion thanks to cationic side chains which form. CS PowderCrystal Riot Control Agent DHE. For reports and publications relating to specific waterbodies visit our Fish Consumption Advisories Publication page.

Largemouth and smallmouth bass. Total Coliform Ecoli MPN Most Probable Number 4000. Oxidation to cobaltIII Compounds of cobalt in the 3 oxidation state exist such as cobaltIII fluoride CoF 3 nitrate CoNO 3 3 and sulfate Co 2 SO 4 3. Cobalt is one of many metals that can be oxidized by nitric acid. CS PowderCrystal Riot Control Agent DHE.
 Source: chemnet.com
Source: chemnet.com
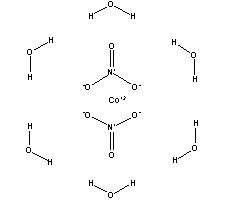
Cobalt II nitrate hexahydrate 99999-Co PURATREM. The other container may be filled with a premixed fertilizer or another general nutrient blend. Cobalt2 nitrate–water 16 AKOS025243254. CO 2 is trapped as Na 2 CO 3 on Ascarite. A flexible redox-active macrocycle enables the electrocatalytic reduction of nitrate to ammonia by a cobalt complex.
 Source: coleparmer.co.uk
Source: coleparmer.co.uk
1 chelating agents are organic compounds which wrap around a metal ion thanks to cationic side chains which form. Page 1 of 1. How many electrons are transferred and what would be the coefficient for H 2O in the balanced reaction. To understand the concerns over contamination in fish and how to reduce your exposure visit our Fish Information page. Co NO 3 H NO H 2O Co 2 a.
 Source: en.wikipedia.org
Source: en.wikipedia.org
None of the above c. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. However cobaltIII chloride CoCl 3 is not stable in normal conditions and would decompose immediately into CoCl 2 and chlorine. The other container may be filled with a premixed fertilizer or another general nutrient blend. Oxidation to cobaltIII Compounds of cobalt in the 3 oxidation state exist such as cobaltIII fluoride CoF 3 nitrate CoNO 3 3 and sulfate Co 2 SO 4 3.
 Source: glentham.com
Source: glentham.com
6 H 2O e. The other container may be filled with a premixed fertilizer or another general nutrient blend. Ionic bonds also melt at high temperatures. 1 OH-and 2 electrons 9. For example Co 2 2 NaOH CoOH 2 2 Na The compound can be prepared by reacting cobaltII nitrate in water with a solution of triethylamine NC 2 H 5 3 as both the base and a complexing agent.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title cobalt 2 nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.